Recovery Test of Sodium Dodecylbenzenesulfonate by TOC-LCPH
In the test method for total organic carbon as listed in the General Tests, Processes and Apparatus section of the Japanese Pharmacopeia (JP), it is specified that the TOC analyzer must be capable of measurement of sample concentrations below 0.050 mg/L, and that the ins t rument achieve a recovery rate of at leas t 0.450 mg/L carbon in measurement of a sodium dodecylbenzenesulfonate solution (0.806 mg/L). Here, we introduce an example of recovery testing with a sodium dodecylbenzenesulfonate solution using the Shimadzu combustion catalytic oxidation model TOCLCPH, and an example of analysis of a sample with a TOC concentration below 0.050 mg/L.
The Japanese Pharmacopeia (JP) specifies that "the decomposition device for either method should be capable of generating not less than 0.450 mg/L of organic carbon when using a solution of sodium dodecylbenzenesulfonate (theoretical value of total organic carbon in this solution is 0.806 mg/L) as the sample."
Because sodium dodecylbenzenesulfonate solution (0.806 mg/ L ) has a carbon concentration of 0.500 mg/L, the instrument must be capable of combustion oxidation sufficient to permit a 90 % detection rate (recovery) when measuring this solution. Thus, we prepared a sodium dodecylbenzenesulfonate solution with a carbon concentration of 0.500 mg/L by dissolving sodium dodecylbenzenesulfonate in distilled water, and then conducted TOC measurement (TC-IC measurement). The results are shown in Fig. 1.
We calibrated the instrument by generating calibration curves using 0 and 1 mgC/ L (1 mg/ L carbon concentration) standard solutions for both the TC and IC measurements. To eliminate the influence of carbon in the distilled water used to prepare the standard solutions for generating both calibration curves, we performed correction using the calibration curve Shift to Origin feature.
Measurement of the sodium dodecylbenzenesulfonate solution with a carbon concentration of 0.500 mg/L yielded a result of 0.498 mg/L (498 μg/L), which is a recovery rate of 99.6 %, thus satisfying the instrument specifications stipulated in the total organic carbon test method of the Japanese Pharmacopeia (JP).
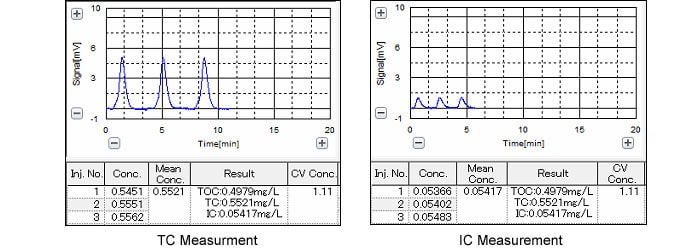
Fig. 1 TOC Measurement Data for 0.500 mg/L (500 μg/L) Sodium Dodecylbenzenesulfonate Solution
TOC Measurement Below 0.050 mg/L
The Japanese Pharmacopeia (JP) specifies that "the instrument should be capable of measuring the amount of organic carbon down to 0.050 mg/L." Thus, we prepared a potassium hydrogen phthalate aqueous solution with a TOC concentration of 0.025 mgC/L.
The results are shown in Fig. 2. Because the distilled water used to prepare the sample includes some slight amount of organic carboncontaining substances, the result of measurement of the 0.025 mgC/L potassium hydrogen phthalate aqueous solut ion was 0.0407 mgC/L, but the coefficient of variation (CV value) was 2.42 %.
This result is near the lower limit of quantitation, and within 10 % of the CV value, demonstrating that the Shimadzu TOC-LCPH combustion catalytic oxidation type TOC analyzer satisfies the TOC measurement criteria value of less than 0.050 mg/L stipulated in the Japanese Pharmacopeia total organic carbon test method.
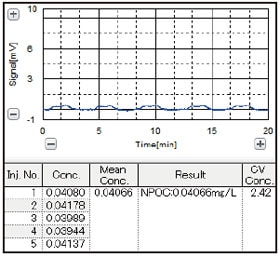
Fig. 2 Data Obtained with Less than 0.05 mg/L (50 µg/L) TOC


