Quantitative Analysis of Trace Impurities in Drugs (LC/MS)
Impurities and Foreign Matter
Quantitative Analysis of Trace Impurities in Drugs (LC/MS)
■ Analysis of Trace Impurities in Atropine
Organic impurities in pharmaceuticals may derive from the drug ingredients, be generated during pharmaceutical production, or be created by decomposition of the drug during storage.
The quantitative analysis of trace impurities in atropine is introduced below.
The results of trace impurity analysis of 50 mg/mL commercially available atropine spiked with 50 ng/mL (0.0001 % ) p-nitrophenol are shown below.
Fig. 1 shows a magnified view of the LC chromatogram and Fig. 2 shows the results of analysis by the LCMS-2020 in SIM mode.
As the concentration is comparatively high at the MS detection sensitivity, the impurity can be easily detected near 4.7 minutes retention time.
The LCMS-2020 permits quantitation of trace impurities down to 0.5 ng/mL content (equivalent to 0.000001 %)
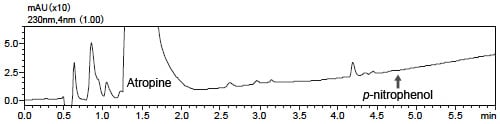
Fig. 1 LC Chromatogram (UV 230 nm)
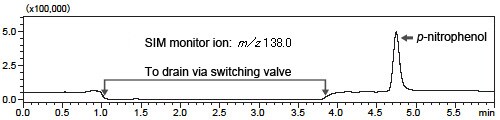
Fig. 2 SIM Chromatogram
Table 1 shows the measurement reproducibility. Quantitation performance was confirmed with CV 7 % max. in the 0.5 to 100 ng/mL range and r2 = 0.999 min.
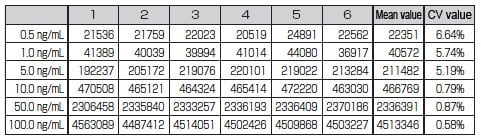
Table 1 p-nitrophenol Measurement Reproducibility
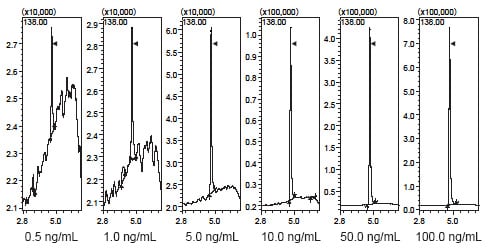
Fig. 3 SIM Chromatogram at Each Concentration
LCMS-2020 High-Performance Liquid Chromatograph Mass Spectrometer

- The detection of impurities over a wide concentration range demands both high sensitivity and a wide dynamic range. The newly developed LCMS-2020 ion optical system, Qarray® Optics, achieves superior sensitivity, reproducibility, and linearity.
- An extensive range of ionization methods is required to handle diverse oil-soluble and water-soluble impurities. The DUIS-2020 is one of the many LCMS-2020 ionization methods. It combines ESI and APCI to ensure that even impurities with significantly different polarities are not missed.


